Plasmology4® is Harnessing the 4th State of Matter
Plasmology4 is a technology company pioneering the emerging field of Plasma Medicine with its proprietary Plaz4 Technology. Plaz4 is a highly advanced and specialized form of non-thermal plasma (physics) that has many potential applications for human healthcare.

What is Non-thermal Plasma?
Non-thermal plasma is ionized gas consisting of positive ions and free electrons, generated in earth atmospheric conditions (atmospheric pressures) at temperatures below 100° C (212° F).

What is Plasma Medicine?
Plasma Medicine is an innovative and emerging field combining plasma science, physics, life science and clinical medicine to advance non-thermal plasma technology for healthcare applications.

Plaz4® Technology
Plaz4 is a novel, non-thermal plasma with advanced characteristics compared to ordinary cold plasmas. As a platform technology, Plaz4 has the potential to be utilized in a wide variety of human health applications. It has the potential to improve certain clinical outcomes, reduce costs and solve many currently unmet needs in healthcare.
The Revolutionary Solution for Health
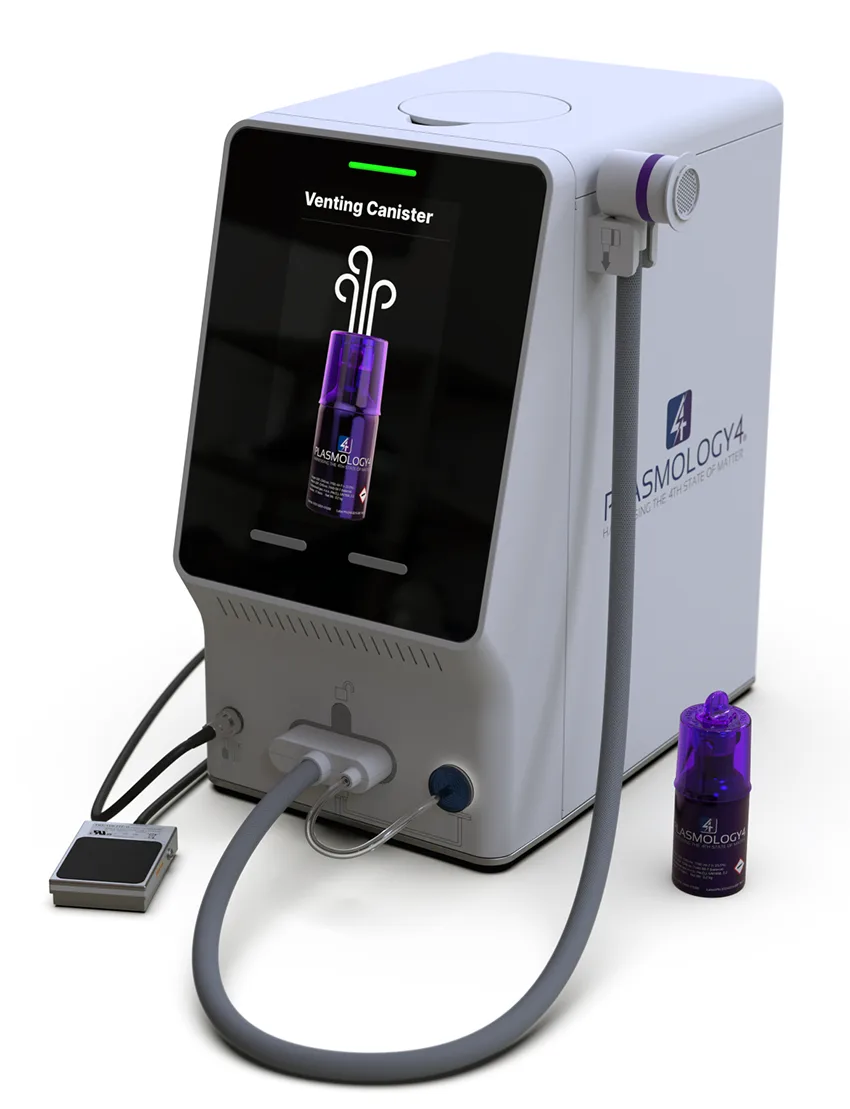
Plasmology4’s first product is the Plaz4 1000 system, which has the potential to address both the worldwide problems of antibiotic-resistant bacteria and non-healing (chronic) wounds. Results from the company’s in vitro studies, and in vivo animal studies, demonstrate that Plaz4 simultaneously kills pathogens (including antibiotic-resistant strains) and heals wounds in a way that is drug-free, painless, fast and cost-effective.
The Plaz4 product has not yet been approved by the US FDA and is not currently for sale.
About the Company
Plasmology4 is a privately held, pre-revenue company headquartered in Scottsdale, Arizona, USA.
Intellectual Property
Plasmology4 has an extensive portfolio of 48 issued patents in the US and Europe with more applications pending. This solidifies our position as a leader in the field of non-thermal plasma and plasma medicine.
Plasmology4 has 48 issued patents in the US and Europe protecting methods of generating and applying non-thermal plasma in various fields.
Designated as a “Breakthrough Technology” by the FDA!
Plasmology4’s first product has been granted into the U.S. Food and Drug Administration’s (FDA) Breakthrough Devices Program for the treatment of “infected wounds, including multidrug refractory bacteria (such as MRSA)”.
The Centers for Disease Control and Prevention (CDC) estimates that more than 2.8 million antibiotic-resistant infections occur in the United States each year, and more than 35,000 people die as a result. These illnesses lead to eight million extra days of hospitalization and over $20 billion in additional healthcare costs. Globally, the CDC estimates there are 700,000 deaths per year that are caused by antibiotic-resistant organisms. By the year 2050, annual deaths are predicted to reach 10 million, adding $100 trillion to healthcare costs. (source: CDC)
The Plaz4 product has not yet been approved by the US FDA and is not currently for sale.
A Breakthrough in Combating Antimicrobial Resistance
Leadership Team Driving Innovation at Plasmology4®

Robert Hummel, MBA
Chief Executive Officer
Robert is a seasoned business executive with 30 years of experience in the medical technology/device industry, with extensive experience in the spine surgery, orthopedic surgery and wound care market segments. His direct experience with identification and assessment of ground-breaking technologies helped to ascertain and validate the early form of what is now known as Plaz4 Technology. Mr. Hummel is an inventor of 20+ patents and patent applications.

Marc Jacofsky, PhD
Chief Scientific Officer
Marc is an expert in the field of biomedical research and is a named inventor on over 30 issued patents. He has managed over 50 clinical trials with over 25 peer-reviewed publications. Marc is also Chief Scientific Officer at The CORE Institute and is Founder and Executive Director of the Musculoskeletal-Orthopedic Research and Education (MORE) Foundation, where he has developed innovative research platforms for the robotic testing of joint replacements and fractures.

Emilia Kulaga, PhD
Senior Director of Research
Emilia is an experienced biomedical researcher with expertise in start-up product development and regulatory strategies. She is currently one of a small group of scientists worldwide developing devices for the therapeutic delivery of cold plasma technologies. Emilia brings invaluable expertise in novel anti-infective products including: nanocomposites, inorganic materials functionalized with biomolecules, drug/antimicrobial agent delivery systems and non-thermal plasmas for medical devices.

Greg Watson
Chief Innovation Officer
Greg has over 30 years of experience in research and development of medical device technologies and is the inventor of the initial form of the Plaz4 technology. He is a named inventor on 25+ patents in the field of cold plasma. His area of research focus is the field of applied electrical and magnetic field energies, with work in electrostatic forces, electron orientation and flow within magnetic flow compression structures, and cold plasma delivery systems.

Michel Yoon
Director of Engineering
Michel is a highly skilled Engineer with 15+ years of experience designing medical devices, with several devices currently on market. His background is in mechanical engineering, industrial design and human factors and specializes in designing high voltage surgical devices, minimally invasive surgical tools, monitoring systems, cardiac implants, and neurostimulation devices. He has 8 issued patents in medical devices.

Rich John, MBA
VP of Information Technology; Accounting and Shareholder Relations
Technology Executive and Integration Specialist with a background in Software
Engineering and Architecture. Responsible for envisioning and aligning strategic
technology goals with business goals. Evaluating and selecting best in class solutions,
planning and overseeing implementation and development projects. Rich has extensive
domain experience in Oil & Gas and Life Sciences and manages a variety of business
functions including HR, Accounting and Investor Relations.

Courtney Versage
Laboratory Manager, Microbiology
A skilled laboratory manager specializing in microbiology, she has dedicated her career to advancing the Plaz4 technology platform, with a strong focus on non-thermal plasma research.

David Jacofsky, MD
Chairman of the Board of Directors
Chairman Scientific Advisory Board


Roy Sanders, MD
Scientific Advisory Board



